Pipeline detail
FM101 (Non-alcoholic steatohepatitis)
| Classification | Detail |
|---|---|
| FM101 |
|
| Indication |
|
| Unmet Needs |
|
| Mechanism of Action |
|
| Efficacy |
|
| Market |
|
Nonalcoholic fatty liver disease(NAFLD) is a disease manifested by triglyceride accumulation in the liver, which sometimes occurs in an individual who does not consume alcohol at all. Viral hepatitis accounted highest confirmed cases of chronic liver disease until approval of effective anti-hepatic viral medications. This makes NAFLD, followed by the increasingly obese population, the most common cause of chronic liver disease.
There are no disease-specific approved drugs for NASH despite the fact that it is a major cause of the liver transplant that cost a high clinical burden. There are high unmet medical needs in F3 and F4 NASH patients as 80% of NASH are manifested by liver cirrhosis and it is correlated with most of the negative outcomes such as liver transplant and mortality. In addition, global regulatory agency such as FDA and EMA suggests guidance for the industry with regard to NASH drug development based on the drug’s ability to resolve hepatic fibrosis that has high clinical correlations with clinical improvements.
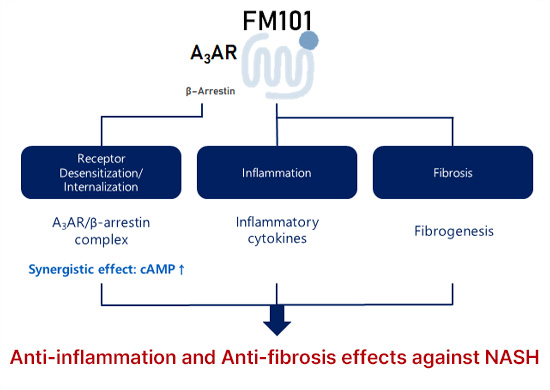
- FM101 mediates biased modulation upon binding A3AR, which suppresses AMP activation leading to an anti-inflammatory/fibrotic effect.
- By inhibiting beta-arrestin, FM101 prevents receptor desensitization and internalization to mediate long term therapeutic effects.




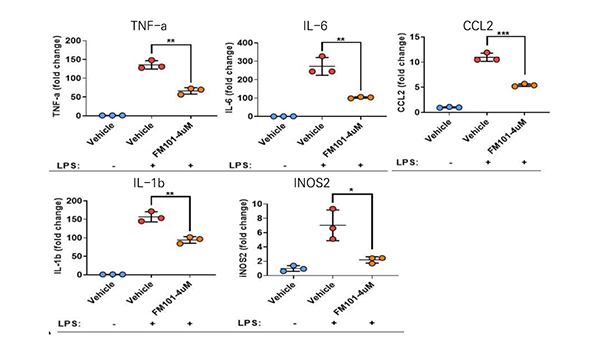
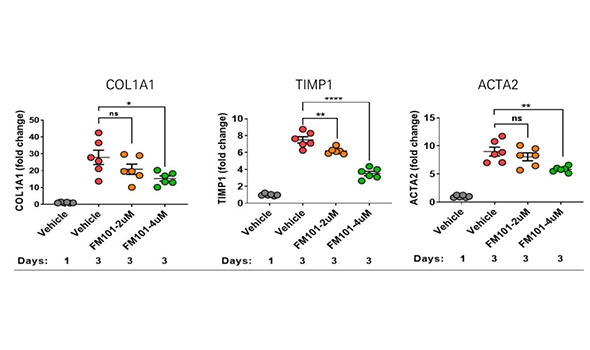
The cells playing critical roles in NASH pathogenesis are ① Kupffer cell (inflammation) and ② Hepatic stellate cells. FM101 significantly reduced inflammatory and fibrotic signaling when Being treated on these cells


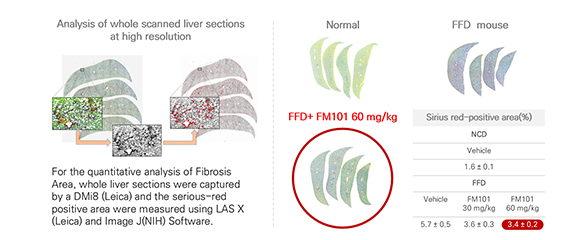
When FM101 is being treated in fast food diet mouse model, liver function (AST/ALT), Inflammatory and fibrotic improvement has been confirmed. This supported our decision to go through Clinical investigation
FM101 reduced inflammatory cytokines in Kupffer cells


FM101 reduced fibrotic cytokines in hepatic stellate cells


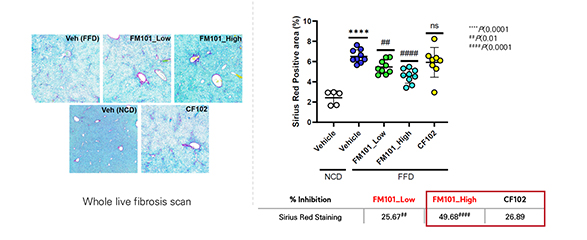
Through whole liver section staining, It has been confirmed that treatment of FM101 reduced area of liver fibrosis upto 56 % compared to FFD control


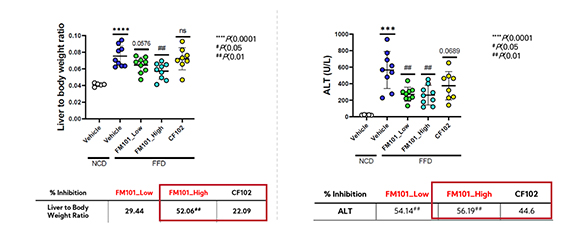
Confirmation of superior efficacy in liver functional improvement compared to CF102
(Left) change in liver weight (Right) change in ALT


Confirmation of superior efficacy in reduction of fibrosis area compared to CF102
FM101 (49.7% reduction) vs CF102 (26.9% reduction)


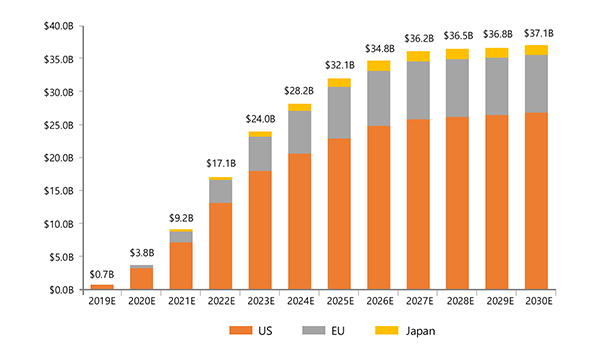
- Current market size (2020): 3.8b$, CAGR: 45%
- Global NASH market forecast: 32.1b$ by the year 2025