Pipeline detail
FM101 (Glaucoma Treatment)
| Classification | Description |
|---|---|
| FM101 |
|
| Indication |
|
| Unmet Needs |
|
| Mechanism of Action |
|
| Efficacy |
|
| Market |
|
Glaucoma, manifested by gradual vision loss due to the weakening of optic nerves caused by various reasons, such as increased intraocular pressure, is often asymptomatic. There are no current methods to restore the weakened nerves effectively. Therefore, clinical management of glaucoma is focused on prevention or delaying vision loss. These include laser, surgery and eye drops.
Currently, prostaglandin-based eye drop is being prescribed as the standard of care for glaucoma. However, It is inconvenient to administer, and side-effects related to active compounds or vehicles in eye drops, such as pigmentation, burning sensation, and conjunctival congestion, have been reported as unmet needs. Therefore, the development of a new drug that is (1) orally available, (2) safe, and (3) Neuroprotective would be of great necessity.


Inconvenience to take Medicine due to tremor (often in elderly patients)



Unresponsive (ex. Normal IOP)


Side-effect such as(pigmentation, burning sensation, and conjunctival congestion) Stop taking medicine Due to cosmetic problem
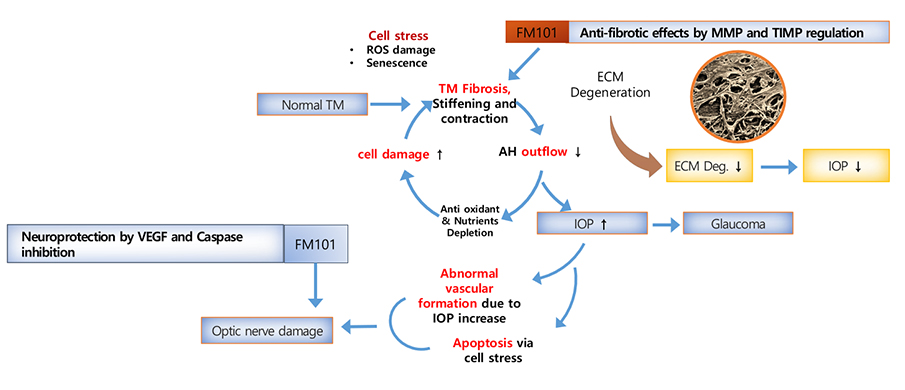
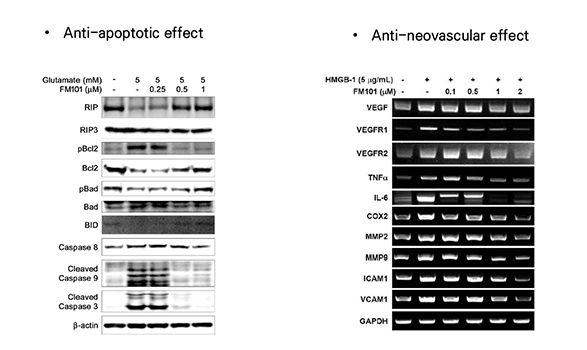
- Anti-inflammatory/fibrotic effect by inhibition of cAMP activation through A3AR biased modulation
- FM101 controls level of the inflammatory and fibrotic genes in the trabecular meshwork(TM), alleviating swollen TM tunnel to increase the outflow of aqueous humor (AH) and eventually decreasing intraocular pressure.
- Neuroprotection is mediated by the A3AR specific signaling pathway, which prevents vision loss.


Evaluation of fibrotic expression(fibronectin) level in TM tissues of OH mouse revealed that the TM tissues of the mice administered with FM101 manifest a similar level of fibrosis expression to the TM of the normal mice.
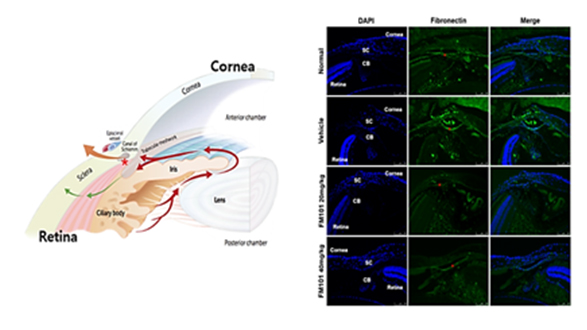


Oral administration of FM101 showed similar or superior efficacy in IOP decrease compared to marketed drugs (Xalatan, Acetazolamide)
- Xalatan: Prostaglandin-based glaucoma eye drop treatment. Several adverse events have been reported
- Acetazolamide: carbonic unhydrase inhibitor, orally availale. However, reported with major adverse event such as anuric renal failures.



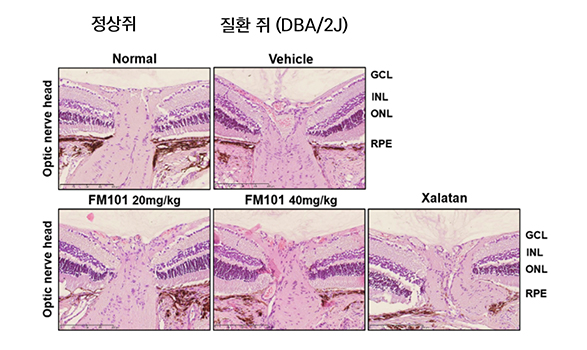
Through evaluation of the optic nerve head tissue section of DBA/2J mouse, it was confirmed that the optic nerve shape of FM101-administered rats was preserved at a level similar to that of normal rats.




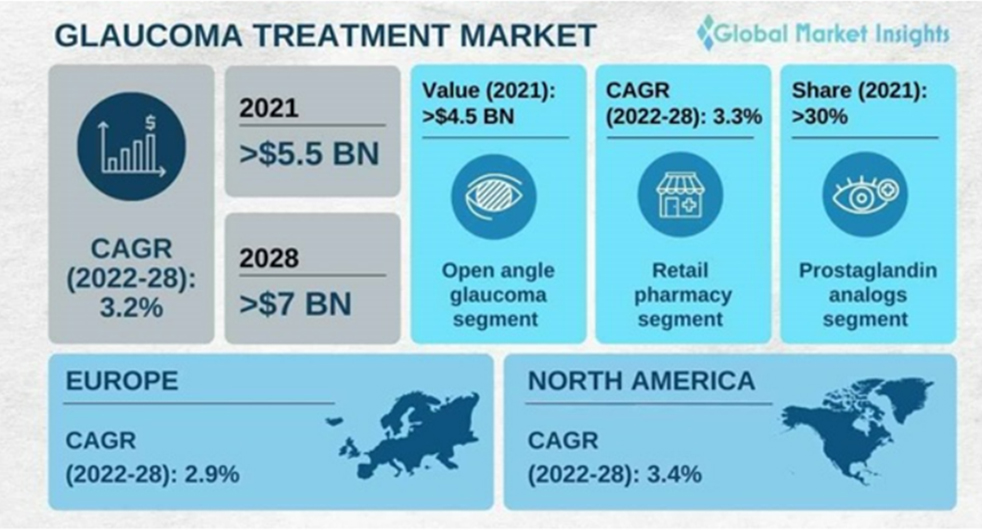
- Glaucoma treatment market size exceeded USD 5.5 billion in 2021 and is expected to witness over 3.2% CAGR from 2022 to 2028. The rising prevalence of glaucoma diseases and the growing demand for advanced therapeutic interventions are some of the major factors that facilitate market growth. According to the World Glaucoma Association, around 76 million people were suffering from glaucoma in 2020.