Pipeline detail
FM101 (Primary biliary cholangitis)
| Classification | Description |
|---|---|
| FM101 |
|
| Indication |
|
| Unmet Needs |
|
| Efficacy |
|
| Market |
|
| Orphan Drug |
|
Indication
Primary Biliary Cholangitis
Primary biliary cholangitis is an autoimmune disease characterized by cholestasis and is a rare disease reported in approximately 1 in 2500 people in North America.
PBC is characterized by the gradual destruction or obstruction of bile ducts within the liver, causing bile to accumulate within the liver. This leads to tissue damage and scarring, or fibrosis, and eventually, cirrhosis
Unmet Needs
Currently, UDCA and Ocaliva are used as first and second-line treatments, but about 40% of patients do not respond to treatment.
The treatment has been reported with severe side effects such as pruritus and chronic fatigue. Hence, new treatments are in high needs.
Efficacy
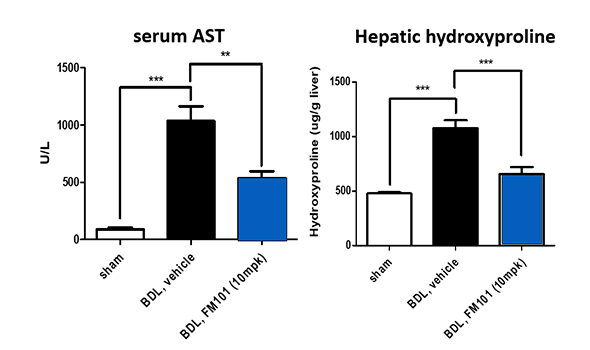


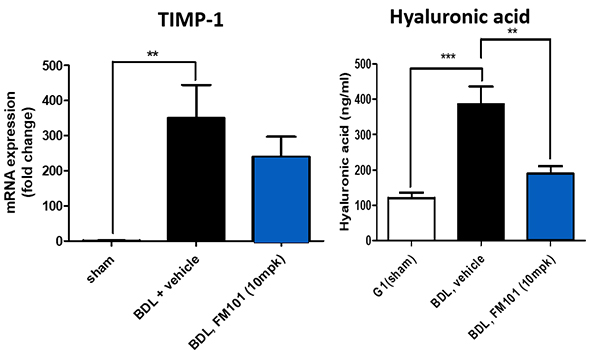








Market
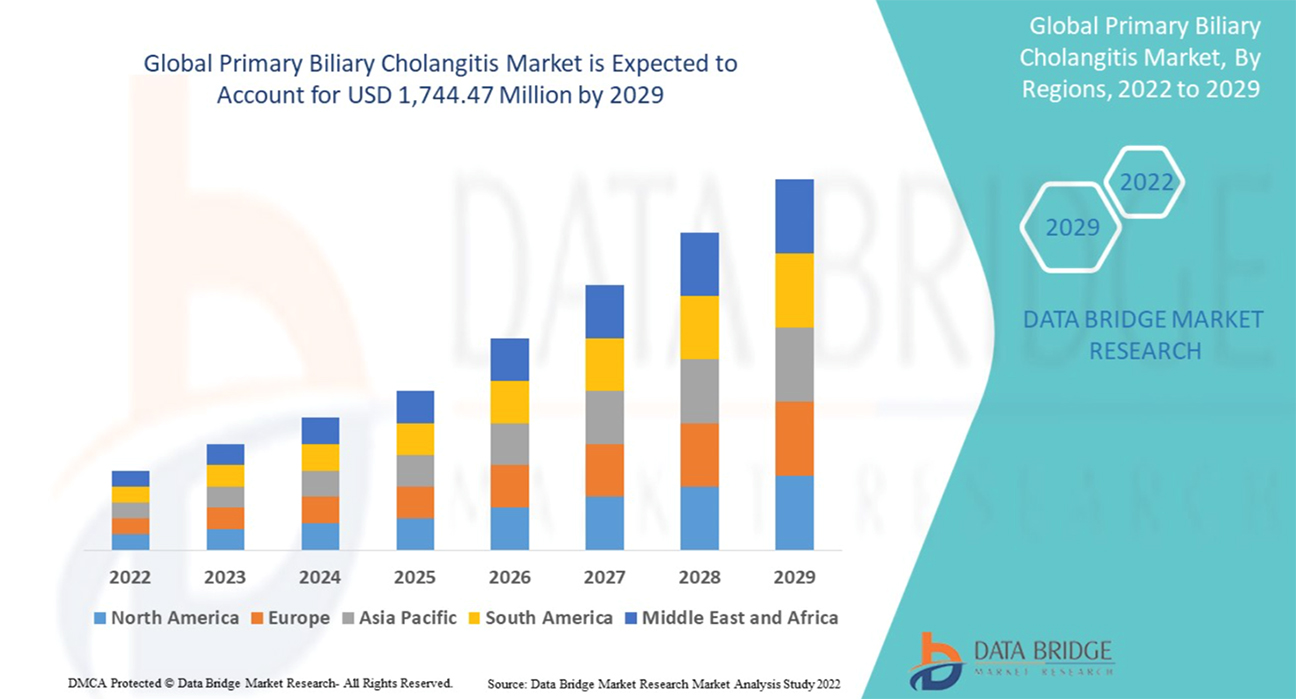


- Compound annual growth rate: 10.5%
- Global PBC Market size : Reaching 1744 m$ by the year 2029



Orphan Drug



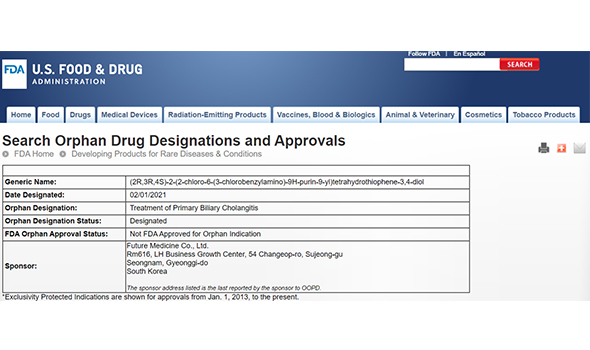


- FM101 was designated as an orphan drug by the USFDA in February 2021.
- The FDA’s orphan drug designation program provides support and benefits through the course of the development and approval of rare, incurable or life-threatening diseases treatment. These include tax exemption (clinical), waiver of PDUFA fee, and 7-year marketing exclusivity










